How safe is America from polio?
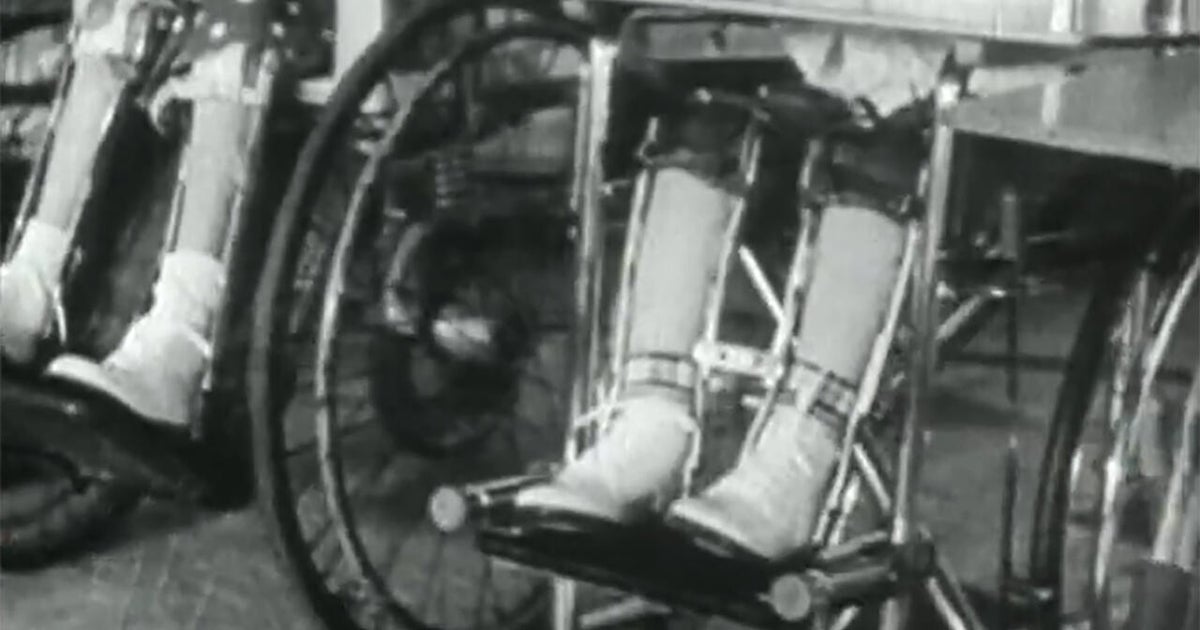
When 13-year-old music prodigy Itzhak Perlman performed on “The Ed Sullivan Show” in 1958, viewers could see his extraordinary talent. What they couldn’t see were the braces and crutches he needed to walk. Perlman was four when he contracted polio. “I was already running and walking, and I remember one morning when I got up…